Author
Research Team
Share
Author
Research Team
Share
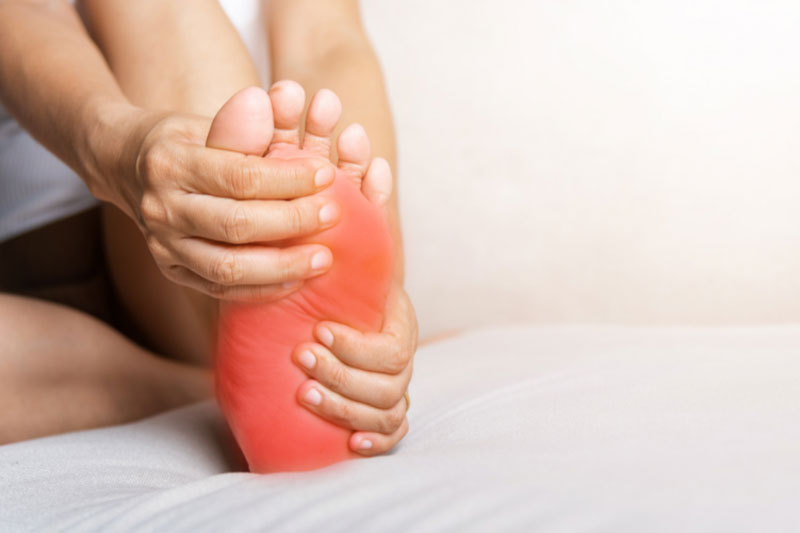
Diabetic peripheral neuropathic pain is a type of nerve pain that affects the feet and legs of people with diabetes. It is caused by high levels of sugar in the blood that damage the nerves over time. The pain may be described as burning, tingling, stabbing, or numbness. If you need additional information, please reach out to our research team.
The purpose of this study is to find out the efficacy of an investigational drug called LX9211 in reducing pain related to diabetic peripheral neuropathy. LX9211 is an investigational drug that is not approved by Health Authorities, including the U.S. FDA.
LX9211 is a new type of investigational drug called an AP2-associated protein kinase 1 inhibitor (AAK1 inhibitor). It is believed that inhibiting AAK1 may help reduce pain caused by diabetic neuropathy.
Who can participate in the PROGRESS study?
You may qualify to participate if you:
▸ Are 18 years of age or older
▸ Have type 1 or type 2 diabetes
▸ Have an A1C less than or equal to 11%
▸ Have ongoing diabetic peripheral neuropathy with moderate to severe pain
Your medical history and other criteria will be checked to see if you can take part in the study.
What is involved in the PROGRESS study?
Approximately 416 participants will be in the study at about 110 research clinics in the United States. The total time you will be in the study will be approximately 16 weeks which includes a Screening Period of up to 2 weeks and Blinded Treatment Period of 14 weeks.
In this study some subjects will receive LX9211, and some will receive placebo. A ‘placebo’ looks like LX9211 but does not have any active substance in it. You will be randomly assigned (like drawing straws) to a treatment group. You will have an equal chance of being in one of the following treatment groups:
▸ Group 1: LX9211 10 mg, once daily
▸ Group 2: LX9211 20 mg, once daily
▸ Group 3: LX9211 20 mg (for 7 days) then 10 mg, once daily
▸ Group 4: Placebo, once daily
Both LX9211 and placebo are referred to as “study drug.” During the study, you will not know if you are receiving LX9211 or placebo. This is the best way to measure the effect of LX9211. You will not be told the treatment group you are assigned to until after the study has ended and the results have been analyzed. Your study doctor can find out the treatment group if there is an emergency or if it is needed to know for your health.
You will be provided with and trained on the use of an electronic diary (e-diary) where you will record information about medications you have taken for pain. You will also complete training on how to accurately report pain on the e-diary.
